X-linked Spinal and Bulbar Muscular Atrophy (Kennedy Disease) via the AR Gene CAG Repeat Expansion
Summary and Pricing 
Test Method
Combination Of Repeat-Primed PCR and Fluorescent Fragment-Length Assay| Test Code | Test Copy Genes | Test CPT Code | Gene CPT Codes Copy CPT Code | Base Price | |
|---|---|---|---|---|---|
| 7501 | AR | 81204 | 81204 | $350 | Order Options and Pricing |
An additional 25% charge will be applied to STAT orders. STAT orders are prioritized throughout the testing process.
Turnaround Time
3 weeks on average for standard orders or 2 weeks on average for STAT orders.
Please note: Once the testing process begins, an Estimated Report Date (ERD) range will be displayed in the portal. This is the most accurate prediction of when your report will be complete and may differ from the average TAT published on our website. About 85% of our tests will be reported within or before the ERD range. We will notify you of significant delays or holds which will impact the ERD. Learn more about turnaround times here.
Targeted Testing
For ordering sequencing of targeted known variants, go to our Targeted Variants page.
Clinical Features and Genetics 
Clinical Features
Spinal and bulbar muscular atrophy (SBMA), also known as Kennedy's disease, is an X-linked neuromuscular disorder caused by degeneration of the lower motor neurons and muscle due to an expanded repeat in the androgen receptor gene. Affected males will typically present between the age of 30 to 50 years of age with muscle weakness, muscle atrophy, and fasciculations (La Spada 2017. PubMed ID: 20301508; Grunseich and Fischbeck. 2015. PubMed ID: 26515625). Other clinical features include gynecomastia, testicular atrophy, and reduced fertility as a result of mild androgen insensitivity. The disease is slowly progressive and eventual involvement of the bulbar muscles typically leads to dysphagia and difficulty with speech articulation (La Spada 2017. PubMed ID: 20301508; Grunseich and Fischbeck. 2015. PubMed ID: 26515625). SBMA is a sex-limited disorder, with females having lower levels of androgen receptor stimulation. Low levels of circulating androgen and X-chromosome inactivation account for the absent symptoms in heterozygous female carriers.
Genetics
Spinal and Bulbar Muscular Atrophy (SBMA) is inherited in an X-linked manner. It is caused by a CAG repeat expansion in the AR gene which occurs in the first exon, and encodes a polyglutamine tract beginning at residue 58 (La Spada et al. 1991. PubMed ID: 2062380). The AR gene is located at Xq11-q12 and encodes the androgen receptor. The repeat copy numbers can be categorized into 4 different categories: < 34 repeats – normal, 35 repeats - unknown, 36-37 – reduced penetrance, > 38 pathogenic allele (La Spada 2017. PubMed ID: 20301508). Repeat lengths of 38-68 CAGs have been reported in patients to date (Grunseich and Fischbeck. 2015. PubMed ID: 26515625; La Spada 2017. PubMed ID: 20301508). The expansion of the polyglutamine tract results in a mutant protein that is toxic to motor neurons and muscle. The toxicity is ligand dependent and likely involves aberrant interaction of the mutant androgen receptor with other nuclear factors, which results in transcriptional dysregulation. The age of onset correlates with length of the CAG repeat, with onset occurring earlier in patients with longer repeats (Atsuta et al. 2006. PubMed ID: 16621916; Grunseich and Fischbeck. 2015). The disease has been widely reported in European, Asian, and American populations.
Clinical Sensitivity - Repeat-Primed PCR & Fragment Length
Clinical sensitivity should be nearly 100% when a patient presents with clinical features of spinal and bulbar muscular atrophy. Targeted mutation analysis, through the combination of the two gene-centered PCR methods (repeat primed and fragment assays) of the AR exon 1 (CAG) repeat region, is predicted to have a nearly 100% detection rate for pathogenic variants. There is a chance that extreme expansions could be missed with the fragment analysis, but the repeat primed PCR expansion assay should identify even extreme expansions.
Testing Strategy
This test consists of a combination of two complementary analyses: (1) a repeat-primed PCR assay, and (2) a fluorescent fragment-length assay.
Repeat-primed PCR Assay The repeat-primed PCR assay is used to determine the presence or absence of a nucleotide repeat expansion, as previously described (Warner et al. 1996. PubMed ID: 9004136; Jama et al. 2013. PubMed ID: 23414820). The PCR is performed with a fluorescently labeled forward primer specific to the gene of interest downstream (3’ assay) of the repeat region. The reverse primer is specific to the CAG repeat region allowing for annealing at multiple locations within the repeat region. Due to the multiple annealing sites, amplicons will vary in size according to the number of nucleotides in the repeat. A tail anchored primer is used in conjunction with the reverse primer to minimize progressive shortening of amplicons. PCR products are then analyzed on an ABI3730xl sequencer to size the PCR products.
Fluorescent Fragment-length Assay PCR analysis using primers that flank the AR exon 1(CAG) repeat allows full length amplification of both normal and expanded alleles. The purpose of this assay is to confirm the results obtained from the repeat primed PCR assay, as well as to help distinguish between individuals homozygous for an allele versus those with one normal sized allele and a second allele containing a large expansion. This procedure also helps to confirm allele sizes in individuals who are heterozygous for two alleles that are very close in repeat number. Expanded repeats have been known to fail to amplify due to their large size. At this time, the largest expansions reported are in the 60-70 repeat range. We anticipate amplification of these alleles, but in cases of extreme expansions, allele drop may be observed due to the size differential. In these cases, the repeat primed PCR assay will provide evidence of a large expansion.
Indications for Test
Male patients with clinical presentation consistent with spinal and bulbar muscular atrophy.
Male patients with clinical presentation consistent with spinal and bulbar muscular atrophy.
Gene
| Official Gene Symbol | OMIM ID |
|---|---|
| AR | 313700 |
| Inheritance | Abbreviation |
|---|---|
| Autosomal Dominant | AD |
| Autosomal Recessive | AR |
| X-Linked | XL |
| Mitochondrial | MT |
Disease
| Name | Inheritance | OMIM ID |
|---|---|---|
| Bulbo-Spinal Atrophy X-Linked | XL | 313200 |
Citations 
Ordering/Specimens 
Ordering Options
We offer several options when ordering sequencing tests. For more information on these options, see our Ordering Instructions page. To view available options, click on the Order Options button within the test description.
myPrevent - Online Ordering
- The test can be added to your online orders in the Summary and Pricing section.
- Once the test has been added log in to myPrevent to fill out an online requisition form.
- PGnome sequencing panels can be ordered via the myPrevent portal only at this time.
Requisition Form
- A completed requisition form must accompany all specimens.
- Billing information along with specimen and shipping instructions are within the requisition form.
- All testing must be ordered by a qualified healthcare provider.
For Requisition Forms, visit our Forms page
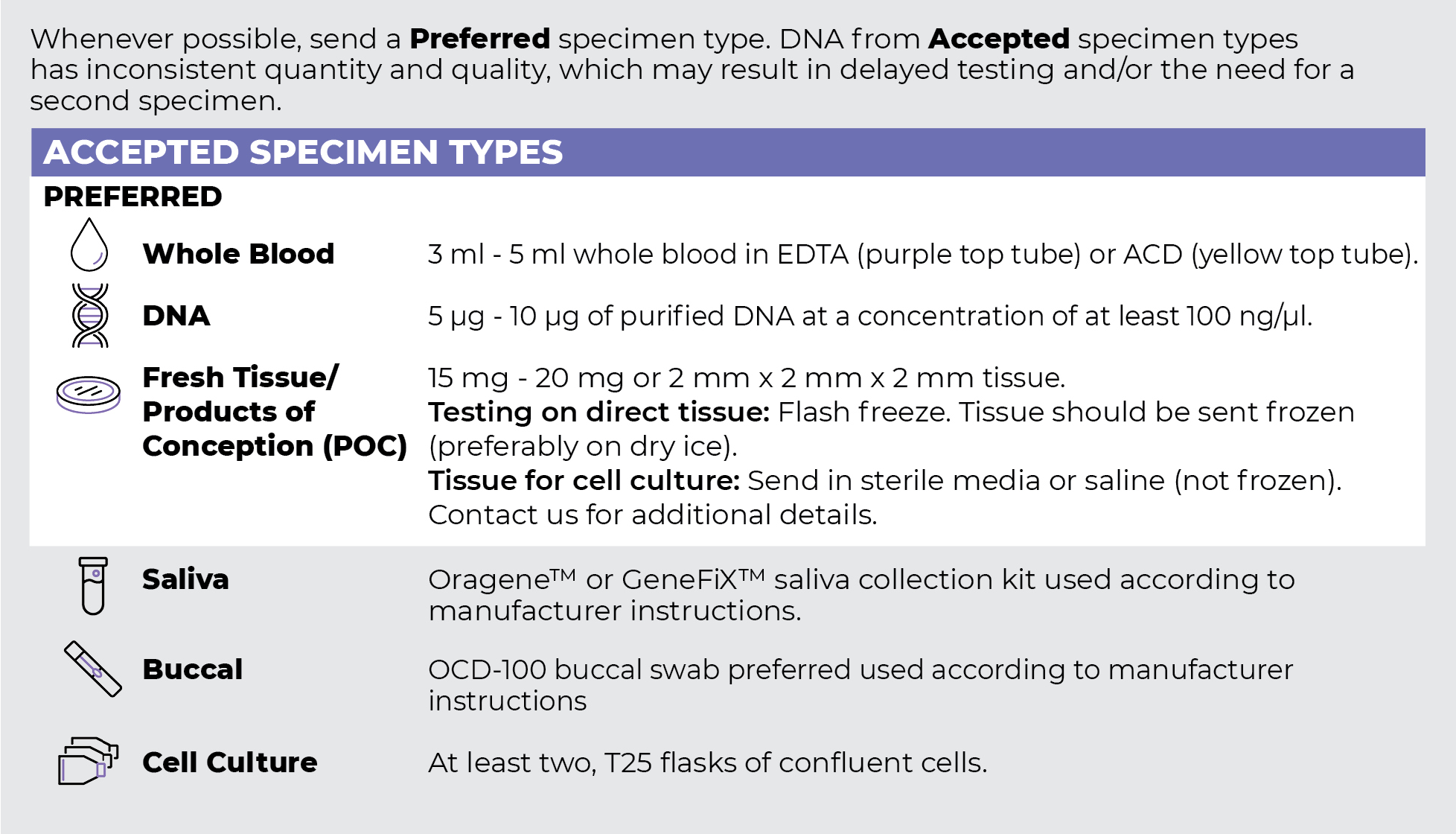
Specimen Types
Specimen Requirements and Shipping Details
ORDER OPTIONS
View Ordering Instructions1) Select Test Type
2) Select Additional Test Options
No Additional Test Options are available for this test.

