Dentatorubral-Pallidoluysian Atrophy (DRPLA) via the ATN1 CAG Repeat Expansion
Summary and Pricing 
Test Method
Combination Of Repeat-Primed PCR and Fluorescent Fragment-Length Assay| Test Code | Test Copy Genes | Test CPT Code | Gene CPT Codes Copy CPT Code | Base Price | |
|---|---|---|---|---|---|
| 5999 | ATN1 | 81177 | 81177 | $350 | Order Options and Pricing |
An additional 25% charge will be applied to STAT orders. STAT orders are prioritized throughout the testing process.
Turnaround Time
3 weeks on average for standard orders or 2 weeks on average for STAT orders.
Please note: Once the testing process begins, an Estimated Report Date (ERD) range will be displayed in the portal. This is the most accurate prediction of when your report will be complete and may differ from the average TAT published on our website. About 85% of our tests will be reported within or before the ERD range. We will notify you of significant delays or holds which will impact the ERD. Learn more about turnaround times here.
Targeted Testing
For ordering sequencing of targeted known variants, go to our Targeted Variants page.
Clinical Features and Genetics 
Clinical Features
Dentatorubral-pallidoluysian atrophy (DRPLA) is a rare progressive neurodegenerative disorder characterized by myoclonus, epilepsy, cerebellar ataxia, choreoathetosis, psychiatric disturbances, and intellectual deterioration or dementia (Veneziano and Frontali. 2016. PubMed ID: 20301664). DRPLA is caused by expansion of a CAG repeat encoding a poly glutamine (Poly-Q) tract in exon 6 on the ATN1 gene. DRPLA is also known as Naito-Oyanagi disease after the researchers that first characterized this disorder in Japan (Kanazawa et al. 1998. PubMed ID: 9933295). It is also referred to Haw River Syndrome after an extended African American kindred from the Haw River area in rural North Carolina (Burke et al. 1994. PubMed ID: 7951323). DRPLA is enriched in in the Japanese population, with an estimated prevalence of 2 to 7 cases per million individuals (Carroll et al. 2018. PubMed ID: 30410817). Outside of Japan, the exact prevalence of DRPLA is presently unknown.
DRPLA displays phenotypic variability as disease severity increases and age of onset decreases with increasing CAG repeat size. Onset varies from early infancy (<1 year) through to the seventh decade with a mean onset of 30 years (Veneziano and Frontali. 2016. PubMed ID: 20301664). Disease duration ranges from 0 to 35 years with an average length of 8 years. Death may occur between 18 to 80 years of age with an average of 49 years. Individuals affected before 20 years of age typically present with ataxia, myoclonus, epilepsy, and progressive intellectual deterioration, while those affected after 20 years of age typically present with ataxia, choreoathetosis, dementia, and psychiatric disturbances (Veneziano and Frontali. 2016. PubMed ID: 20301664). In some cases, brain neuroimaging may reveal progressive cerebellar and brain stem atrophy (Tsuji et al. 2012. PubMed ID: 21827919). Treatment is limited to managing individual symptoms such as antiepileptic drugs, psychotropic medications, and riluzole and rehabilitation therapy for epilepsy, psychiatric manifestations, and ataxia, respectively (Veneziano and Frontali. 2016. PubMed ID: 20301664). The clinical features and presentation of DRLPA displays overlap with hereditary ataxias and Huntington disease (Brusco et al. 2004. PubMed ID: 15148151; Veneziano and Frontali. 2016. PubMed ID: 20301664). Genetic testing may aide in establishing a differential diagnosis. Genetic testing may also assist reproductive planning.
Genetics
DRLPA is inherited in an autosomal dominant manner. It is caused by a CAG repeat expansion in the ATN1 gene that occurs in exon six and encodes a polglutamine tract beginning at residue 488. Repeat copy numbers can be categorized into three categories: 6-35 CAG repeats are normal alleles, 36-47 CAG repeats are mutable normal alleles, and ≥ 48 CAG repeats are full penetrance pathogenic alleles (Veneziano and Frontali. 2016. PubMed ID: 20301664). Mutable normal alleles are unstable and may expand upon transmission to subsequent generations. Although not typically associated with symptoms, a limited number of cases with a mild DRPLA phenotype and repeat lengths in the mutable normal allele range have been reported (Carroll et al. 2018. PubMed ID: 30410817). The mutable and pathogenic CAG repeats are known to exhibit anticipation as the repeat expands upon transmission to successive generations with earlier age of onset and more severe course of disease. Expansion of the CAG repeat is typically associated with paternal transmission, but may occur with maternal transmission in rare instances (Nagafuchi et al. 1994. PubMed ID: 8136826; Veneziano and Frontali. 2016. PubMed ID: 20301664).
The ATN1 gene encodes the atrophin-1 protein, a transcriptional corepressor involved in nuclear signaling that is widely expressed in the central nervous system and other tissues (Wang et al. 2008. PubMed ID: 17150957). Although the function of the ATN1 protein is yet to be fully elucidated, animal models provide insight into the pathologic process. Mice with a single 129 CAG repeat allele in the ATN1 gene display a progressive neurodegenerative phenotype similar to juvenile onset DRPLA (Sato et al. 2009. PubMed ID: 19039037). These mice were observed to have progressive brain atrophy that was associated with neuronal intranuclear accumulation of the elongated ATN1 protein (Sato et al. 2009. PubMed ID: 19039037). This protein accumulation leads to the formation of neuronal intranuclear inclusions, atrophy of the perikarya and dendrites, and a decrease in the number and size of dendritic spines, which leads to brain atrophy and neuronal dysfunction without cell death (Saki et al. 2006. PubMed ID: 16891319).
While the ATN1 CAG repeat expansion is exclusively associated with DRPLA, de novo pathogenic missense variants and in-frame insertions in the ATN1 gene are also reported to be associated with autosomal dominant congenital hypotonia, epilepsy, developmental delay, and digital anomalies (Palmer et al. 2019. PubMed ID: 30827498). Please see our Custom Panel tool if you are interested in testing for these additional pathogenic variants.
Clinical Sensitivity - Repeat-Primed PCR & Fragment Length
This test is designed to identify the number of CAG repeats in the two alleles for ATN1. Clinical sensitivity is nearly 100% when a patient presents with symptoms and a familial history of the disease (Veneziano and Frontali. 2016. PubMed ID: 20301664).
Testing Strategy
This test consists of a combination of three complementary PCR assays that are used as a screening method for the presence or absence of the CAG trinucleotide repeat expansion located in the sixth exon of the ATN1 gene. These assays include two repeat-primed PCR assays for the 3’ and 5’ ends of the repeat region, as well as a fluorescent fragment-length assay (also known as the flanking assay) (Warner et al. 1996. PubMed ID: 9004136; DeJesus-Hernandez et al. 2011. PubMed ID: 21944778; Renton et al. 2011. PubMed ID: 21944779; Cleary et al. 2016. PubMed ID: 27288208). Allele sizing only includes pure CAG repeats. There are four glutamine residues prior to the poly glutamine CAG expansion (sequence is CAGCAACAGCAA[CAG]N), which are not counted in our allele sizing assays. This is consistent with ATN1 CAG repeat expansion reporting in the literature.
There is a chance that highly expanded alleles (>100) may not be detectable by fragment analysis. However, the repeat primed PCR expansion assays should detect highly expanded alleles.
Indications for Test
Candidates for this test are patients with clinical features suggestive of DRPLA. This test is also indicated for family members of patients who have the CAG trinucleotide repeat expansion in ATN1.
Candidates for this test are patients with clinical features suggestive of DRPLA. This test is also indicated for family members of patients who have the CAG trinucleotide repeat expansion in ATN1.
Gene
| Official Gene Symbol | OMIM ID |
|---|---|
| ATN1 | 607462 |
| Inheritance | Abbreviation |
|---|---|
| Autosomal Dominant | AD |
| Autosomal Recessive | AR |
| X-Linked | XL |
| Mitochondrial | MT |
Disease
| Name | Inheritance | OMIM ID |
|---|---|---|
| Dentatorubral Pallidoluysian Atrophy | AD | 125370 |
Citations 
- Brusco et al. 2004. PubMed ID: 15148151
- Burke et al. 1994. PubMed ID: 7951323
- Carroll et al. 2018. PubMed ID: 30410817
- Cleary et al. 2016. PubMed ID: 27288208
- DeJesus-Hernadez et al. 2011. PubMed ID: 21944778
- Kanazawa. 1998. PubMed ID: 9933295
- Nagafuchi et al. 1994. PubMed ID: 8136826
- Palmer et al. 2019. PubMed ID: 30827498
- Renton et al. 2011. PubMed ID: 21944779
- Sakai et al. 2006. PubMed ID: 16891319
- Sato et al. 2009. PubMed ID: 19039037
- Tsuji. 2012. PubMed ID: 21827919
- Veneziano and Frontali. 2016. PubMed ID: 20301664
- Wang et al. 2008. PubMed ID: 17150957
- Warner et al. 1996. PubMed ID: 9004136
Ordering/Specimens 
Ordering Options
We offer several options when ordering sequencing tests. For more information on these options, see our Ordering Instructions page. To view available options, click on the Order Options button within the test description.
myPrevent - Online Ordering
- The test can be added to your online orders in the Summary and Pricing section.
- Once the test has been added log in to myPrevent to fill out an online requisition form.
- PGnome sequencing panels can be ordered via the myPrevent portal only at this time.
Requisition Form
- A completed requisition form must accompany all specimens.
- Billing information along with specimen and shipping instructions are within the requisition form.
- All testing must be ordered by a qualified healthcare provider.
For Requisition Forms, visit our Forms page
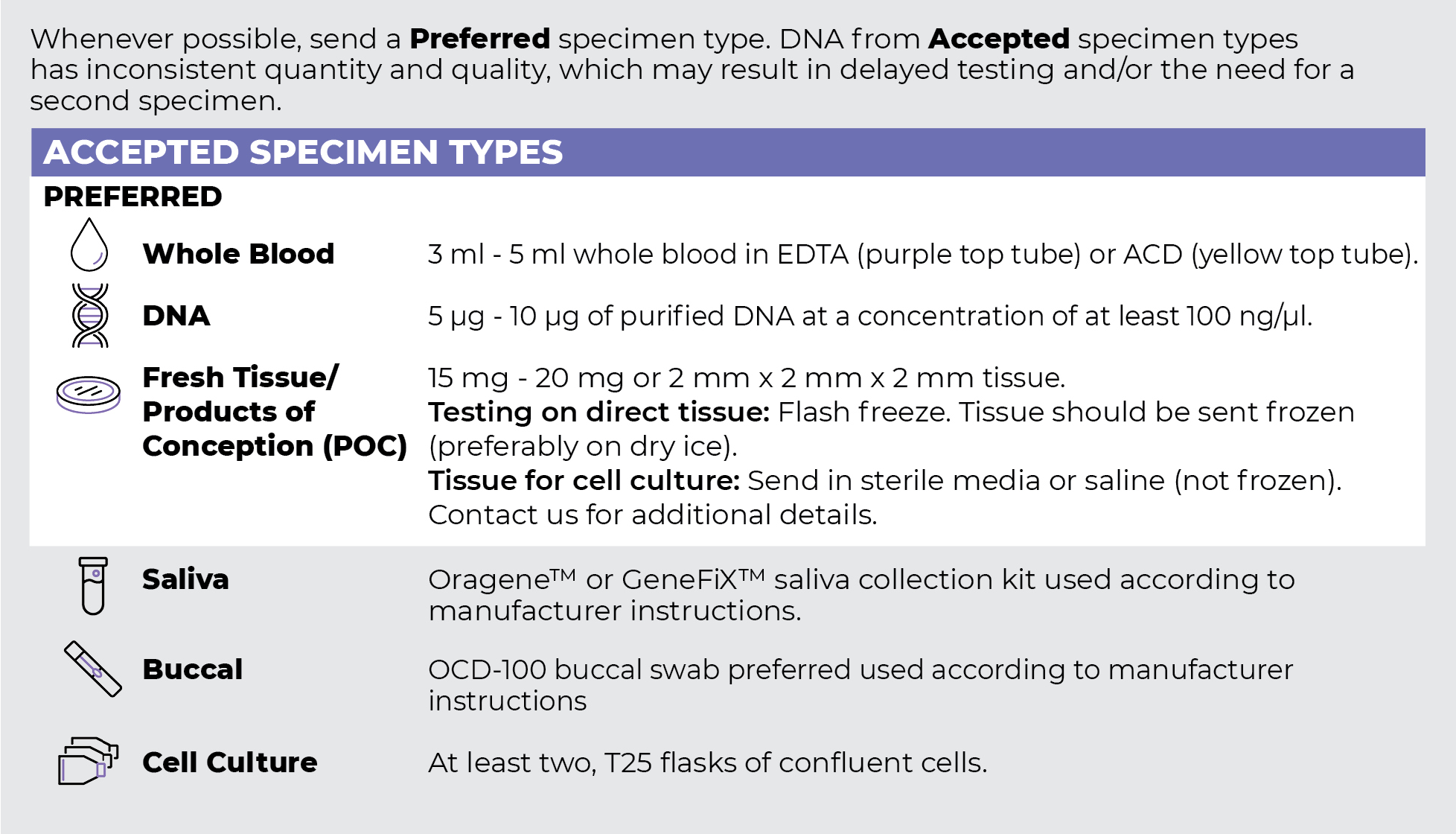
Specimen Types
Specimen Requirements and Shipping Details
ORDER OPTIONS
View Ordering Instructions1) Select Test Type
2) Select Additional Test Options
No Additional Test Options are available for this test.

