Spinocerebellar Ataxia Type 2 via the ATXN2 CAG Repeat Expansion
Summary and Pricing 
Test Method
Combination Of Repeat-Primed PCR and Fluorescent Fragment-Length Assay| Test Code | Test Copy Genes | Test CPT Code | Gene CPT Codes Copy CPT Code | Base Price | |
|---|---|---|---|---|---|
| 12976 | ATXN2 | 81179 | 81179 | $350 | Order Options and Pricing |
An additional 25% charge will be applied to STAT orders. STAT orders are prioritized throughout the testing process.
Turnaround Time
3 weeks on average for standard orders or 2 weeks on average for STAT orders.
Please note: Once the testing process begins, an Estimated Report Date (ERD) range will be displayed in the portal. This is the most accurate prediction of when your report will be complete and may differ from the average TAT published on our website. About 85% of our tests will be reported within or before the ERD range. We will notify you of significant delays or holds which will impact the ERD. Learn more about turnaround times here.
Targeted Testing
For ordering sequencing of targeted known variants, go to our Targeted Variants page.
Clinical Features and Genetics 
Clinical Features
Expansion of the poly-glutamine track in ATXN2 has been associated with several conditions including spinocerebellar ataxia 2 (SCA2), amyotrophic lateral sclerosis (ALS), and progressive encephalopathy with autonomic dysfunction (Pulst. 2019. PubMed ID: 20301452; Sproviero et al. 2017. PubMed ID: 28017481; Paciorkowski et al. 2011. PubMed ID: 21880993).
Spinocerebellar ataxia type 2 (SCA2) is an autosomal dominant form of SCA. All individuals with SCA2 demonstrate cerebellar dysfunction characterized by progressive ataxia and dysarthria (Pulst et al. 1996. PubMed ID: 8896555). Slow saccadic eye movements and peripheral neuropathy are also observed in the majority of patients. Minor clinical features observed in subsets of patients include myoclonus, dystonia, chorea, pyramidal involvement, and intellectual disability (Geschwind et al. 1997. PubMed ID: 9106530; Pulst et al. 1996. PubMed ID: 8896555; Schöls et al. 1997. PubMed ID: 9403486). Progressive encephalopathy, global developmental delay, and hypotonia presenting within the first year of life have been reported in a few cases with expansions >200 repeats. Other variable features include retinitis pigmentosa, optic nerve atrophy, cortical visual impairment, infantile spasms, and seizures (Paciorkowski et al. 2011. PubMed ID: 21880993).
The mean age of disease onset of SCA2 is in the fourth decade of life. There is some correlation between the number of CAG repeats and age of onset. Age of onset varies from 20 to 60 years of age in individuals with 37 CAG repeats (Pulst et al. 1996. PubMed ID: 8896555). In individuals with greater than 45 repeats, age of onset is typically before 20 years of age (Imbert et al. 1996. PubMed ID: 8896557; Pulst et al. 1996. PubMed ID: 8896555; Sanpei. 1996. PubMed ID: 8896556; Cancel et al. 1997. PubMed ID: 9158145; Geschwind et al. 1997. PubMed ID: 9106530). However in about half of cases, CAG repeat length is not associated with age of onset (Figueroa et al. 2017. PubMed ID: 28534046). SCA2 has been shown to demonstrate anticipation in families. In other words, the phenotype severity increases and age of onset decreases from parent to child as a result of expansion of the CAG repeats in subsequent generations (Pulst. 2019. PubMed ID: 20301452).
SCA2 is the second most common subtype of autosomal dominant cerebellar ataxias worldwide. The overall incidence is estimated to 1-2 in 100,000 globally. In Cuba, the incidence is about 6-7 in 100,000 due to a founder effect (Velázquez-Pérez et al. 2017. PubMed ID: 28955296).
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by motor neuron impairment in the cortex, brain stem, and spinal cord (Hardiman et al. 2017. PubMed ID: 28980624). The dysfunction and loss of these neurons results in rapid progressive muscle weakness, atrophy and ultimately paralysis of limb, bulbar and respiratory muscles. About 50% of patients also develop cognitive and behavioral impairment and another 13% developing frontotemporal dementia (van Es et al. 2017. PubMed ID: 28552366). The mean age of symptom onset is 55 years of age for familial cases and 65 years of age for sporadic cases; most cases begin between 40 and 70 years of age. The annual incidence of ALS is 2-3 per 100,000 (van Es et al. 2017. PubMed ID: 28552366; Brown and Al-Chalabi. 2017. PubMed ID: 28700839; Siddique et al. 2019. PubMed ID: 20301623).
Genetics
The ATXN2 gene is expressed in the nervous system and is involved in mRNA processing and regulating protein translation (Yokoshi et al. 2014. PubMed ID: 24954906). Expansions of the poly glutamine (CAG) track in exon 1 are associated with autosomal dominant SCA2 and progressive encephalopathy and increased ALS risk. Alleles with 31 or fewer CAG repeats are not disease causing, though a recessive form of SCA2 has been reported in one individual homozygous for 31 repeats (Tojima et al. 2018. PubMed ID: 30533529). The presence of 32 repeats is uncommon and is considered an allele of uncertain significance for SCA2 (Pulst. 2019. PubMed ID: 20301452). The presence of 33 or more CAG repeats are considered to be in the pathogenic range, though 33-34 repeats are reported to have reduced penetrance. Individuals with 33-34 repeats may not develop the disease, or they may have later disease onset (Riess et al. 1997. PubMed ID: 10735276). The most common disease causing alleles contain 37 to 39 repeats, but expansions containing greater than 200 repeats have been reported (Babovic-Vuksanovic et al. 1998. PubMed ID: 9779806; Pulst. 2019. PubMed ID: 20301452). Interruptions of CAG repeats with CAA, which also codes for glutamine, does not reduce the pathogenicity of the overall repeat size, though it may stabilize the repeat size and reduce the ability of the allele to expand in subsequent generations (Choudhry et al. 2001. PubMed ID: 11689490; Costanzi-Porrini et al. 2000. PubMed ID: 10668721).
Multiple mouse models have been generated to study SCA2 disease onset and progression (Alves-Cruzeiro et al. 2016. PubMed ID: 28018166). ATXN2 is not considered essential for viability in tissue culture cells (Rancati et al. 2018. PubMed ID: 29033457), and mice with constitutive deletion of Atxn2 were viable with no apparent developmental defects (Lastres-Becker et al. 2008. PubMed ID: 18250099).
Expansions ranging from 29-32 repeats are associated with an increased risk for development of ALS. While expansions >32 repeats are typically associated with SCA2, patients with ALS and with >32 repeats have been reported (Sproviero et al. 2017. PubMed ID: 28017481; Ross et al. 2011. PubMed ID: 21610160). ALS has also been reported in a family with one affected individual being homozygous for 33 repeats and a second affected individual being compound heterozygous for 31 and 33 repeats (Van Damme et al. 2011. PubMed ID: 21562247). Intermediate expansions of 29-32 repeats are interpreted as risk alleles for ALS as expansions up to 31 repeats have also been reported in controls (Van Damme et al. 2011. PubMed ID: 21562247). 25-40% of familial ALS cases in American and Europeans are due to repeat expansions in C9orf72. Autosomal dominant pathogenic variants in the SOD1, TARDBP, and FUS accounting for about 15%, 4%, and 5% of familial ALS cases (Brown and Al-Chalabi. 2017. PubMed ID: 28700839; Siddique et al. 2019. PubMed ID: 20301623).
Clinical Sensitivity - Repeat-Primed PCR & Fragment Length
This test for repeat expansions in ATXN2 has near 100% clinical sensitivity for detection of SCA2 (Pulst. 2019. PubMed ID: 20301452).
In a combined study of 1,474 British and 1,328 Dutch individuals with ALS, individuals with an expansion between 29-32 repeats were at increased risk for ALS (odds ratio 3.06 [95% CI 2.37-3.94; p=6x10-18). Relative risk for ALS is increased from 1.61 for 29 repeats to 8.08 for 32 repeats. No association was found between repeat length and age of onset or survival (Sproviero et al. 2017. PubMed ID: 28017481). In a separate study, >30 repeats was shown to increase risk for ALS (odds ratio 5.57 [95%CI 1.95-15.88]; p=0.001) (Ross et al. 2011. PubMed ID: 21610160).
Clinical sensitivity for progressive encephalopathy with autonomic dysfunction is unknown due to the limited cases reported.
Testing Strategy
This test is designed to detect pathogenic expansions of the poly-glutamine CAG repeat in exon 1 of ATXN2. Our assay is similar to an assay described by Van Damme et al. (Van Damme et al. 2011. PubMed ID: 21562247). A combination of amplicon-length analysis and repeat-primed PCR is used to determine the numbers of CAG repeats. Two, separate repeat-primed PCR assays, one for both the 3' and 5' ends of the repeat region, are used. With our assay, we can accurately determine the numbers of repeats in alleles up to about 40 repeats. Alleles of more than 40 repeats can be readily detected by our repeat primed PCR, but the exact number of repeats cannot be accurately determined. Our assay does not assess for CAA interruptions.
Indications for Test
Testing for ATXN2 repeat expansions is recommended for individuals suspected to have SCA2 (presentation of progressive ataxia and dysarthria, nystagmus, and slow saccadic eye movement) in addition to a family history consistent with autosomal dominant inheritance.
Testing for ATXN2 repeat expansions is recommended for individuals suspected to have SCA2 (presentation of progressive ataxia and dysarthria, nystagmus, and slow saccadic eye movement) in addition to a family history consistent with autosomal dominant inheritance.
Gene
| Official Gene Symbol | OMIM ID |
|---|---|
| ATXN2 | 601517 |
| Inheritance | Abbreviation |
|---|---|
| Autosomal Dominant | AD |
| Autosomal Recessive | AR |
| X-Linked | XL |
| Mitochondrial | MT |
Diseases
| Name | Inheritance | OMIM ID |
|---|---|---|
| Parkinson's Disease | AD | 168600 |
| Spinocerebellar Ataxia 2 | AD | 183090 |
Related Test
| Name |
|---|
| Parkinson Disease Panel |
Citations 
- Alves-Cruzeiro et al. 2016. PubMed ID: 28018166
- Babovic-Vuksanovic et al. 1998. PubMed ID: 9779806
- Brown and Al-Chalabi. 2017. PubMed ID: 28700839
- Cancel et al. 1997. PubMed ID: 9158145
- Choudhry et al. 2001. PubMed ID: 11689490
- Costanzi-Porrini et al. 2000. PubMed ID: 10668721
- Figueroa et al. 2017. PubMed ID: 28534046
- Geschwind et al. 1997. PubMed ID: 9106530
- Hardiman et al. 2017. PubMed ID: 28980624
- Imbert et al. 1996. PubMed ID: 8896557
- Lastres-Becker et al. 2008. PubMed ID: 18250099
- Paciorkowski et al. 2011. PubMed ID: 21880993
- Pulst et al. 1996. PubMed ID: 8896555
- Pulst. 2019. PubMed ID: 20301452
- Rancati et al. 2018. PubMed ID: 29033457
- Riess et al. 1997. PubMed ID: 10735276
- Ross et al. 2011. PubMed ID: 21610160
- Sanpei et al. 1996. PubMed ID: 8896556
- Schöls et al. 1997. PubMed ID: 9403486
- Siddique et al. 2019. PubMed ID: 20301623
- Sproviero et al. 2017. PubMed ID: 28017481
- Tojima et al. 2018. PubMed ID: 30533529
- Van Damme et al. 2011. PubMed ID: 21562247
- van Es et al. 2017. PubMed ID: 28552366
- Velázquez-Pérez et al. 2017. PubMed ID: 28955296
- Yokoshi et al. 2014. PubMed ID: 24954906
Ordering/Specimens 
Ordering Options
We offer several options when ordering sequencing tests. For more information on these options, see our Ordering Instructions page. To view available options, click on the Order Options button within the test description.
myPrevent - Online Ordering
- The test can be added to your online orders in the Summary and Pricing section.
- Once the test has been added log in to myPrevent to fill out an online requisition form.
- PGnome sequencing panels can be ordered via the myPrevent portal only at this time.
Requisition Form
- A completed requisition form must accompany all specimens.
- Billing information along with specimen and shipping instructions are within the requisition form.
- All testing must be ordered by a qualified healthcare provider.
For Requisition Forms, visit our Forms page
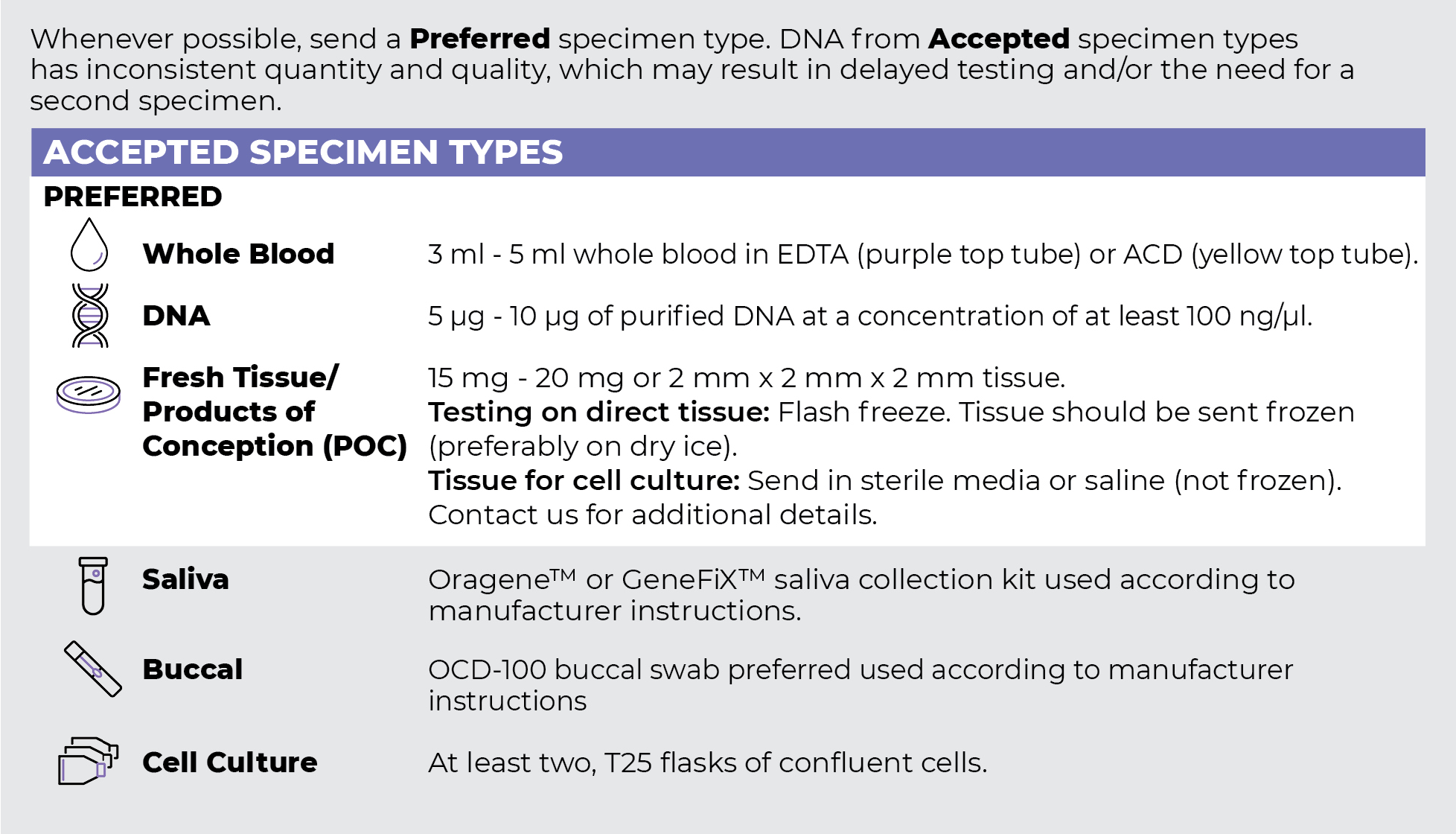
Specimen Types
Specimen Requirements and Shipping Details
ORDER OPTIONS
View Ordering Instructions1) Select Test Type
2) Select Additional Test Options
No Additional Test Options are available for this test.

