Oculopharyngeal Muscular Dystrophy via the PABPN1 (GCN) Repeat Expansion
Summary and Pricing 
Test Method
Combination of Sanger Sequencing and Fluorescent Fragment-Length Assay| Test Code | Test Copy Genes | Test CPT Code | Gene CPT Codes Copy CPT Code | Base Price | |
|---|---|---|---|---|---|
| 6058 | PABPN1 | 81312 | 81312 | $390 | Order Options and Pricing |
An additional 25% charge will be applied to STAT orders. STAT orders are prioritized throughout the testing process.
Turnaround Time
The great majority of tests are completed within 4 weeks.
Please note: Once the testing process begins, an Estimated Report Date (ERD) range will be displayed in the portal. This is the most accurate prediction of when your report will be complete and may differ from the average TAT published on our website. About 85% of our tests will be reported within or before the ERD range. We will notify you of significant delays or holds which will impact the ERD. Learn more about turnaround times here.
Targeted Testing
For ordering sequencing of targeted known variants, go to our Targeted Variants page.
Clinical Features and Genetics 
Clinical Features
Oculopharyngeal muscular dystrophy (OPMD) is a disorder characterized by slowly progressive ptosis and dysphagia. Onset of clinical features usually occurs in the 4th to 6th decade, although this can vary (Brais et al. 1998. PubMed ID: 9462747; van der Sluijs et al. 2003. PubMed ID: 12673802; Trollet et al. 2014. PubMed ID: 20301305; Richard et al. 2017. PubMed ID: 28011929). Additional symptoms, which typically occur later in the disease, can include tongue weakness, proximal limb weakness, dysphonia, wet voice due to pooling of saliva, limitations in upward gaze, and facial muscle weakness (Trollet et al. 2014. PubMed ID: 20301305; van der Sluijs et al. 2016. PubMed ID: 27854203). Lifespan does not seem to be affected in most patients, although quality of life may be reduced (Blumen et al. 2009. PubMed ID: 19704078).
A subset of patients present with severe, early-onset OPMD or later-onset milder OPMD. These types of clinical presentations are associated with different pathological variations in the PABPN1 gene (see Genetics section, below). Severely affected patients see an onset of ptosis and dysphagia prior to 45 years of age. Such patients may also present with incapacitating leg muscle weakness that occurs prior to 60 years of age, and may require the use of a wheelchair (Blumen et al. 2009. PubMed ID: 19704078; Trollet et al. 2014. PubMed ID: 20301305; Richard et al. 2017. PubMed ID: 28011929). Patients with a milder form of OPMD may see an onset of clinical symptoms in the 6th or 7th decade, with reduced severity (Trollet et al. 2014. PubMed ID: 20301305; Richard et al. 2017. PubMed ID: 28011929).
A muscle biopsy of an OPMD patient reveals characteristic intranuclear inclusions (INIs) in skeletal muscle, long considered the pathological hallmark feature of this disorder (Trollet et al. 2014. PubMed ID: 20301305; van der Sluijs et al. 2016. PubMed ID: 27854203). The inclusions are comprised of tubular filaments that are up to 250 nm in length, 8.5 nm in external diameter, and 3 nm in internal diameter. Such filaments are observed in ~2-15% of myonuclei, depending upon the detection technique utilized (Trollet et al. 2014. PubMed ID: 20301305). It should be noted, however, that muscle biopsy is no longer recommended for diagnosis unless a patient with clinical features of OPMD is found to have two normal PABPN1 alleles (Trollet et al. 2014. PubMed ID: 20301305).
Treatment of OPMD patients is currently focused on management of symptoms via dietary and lifestyle changes, as well as surgical methods to address ptosis and dysphagia (Trollet et al. 2014. PubMed ID: 20301305).
Of note, two recent studies have shown linkage, in Bukharan Jewish patients, between an OMPD full penetrance allele (see Genetics section) and a pathogenic variant in the nearby NRL gene (Newman et al. 2016. PubMed ID: 27732723; Braverman et al. 2017. PubMed ID: 28590779). The reported patients homozygous for both the PABPN1 and NRL variants presented with early-onset OPMD as well as severe, slowly progressive visual loss including night blindness and reduced visual acuity.
Genetics
OPMD is an autosomal dominant disorder. It has been reported in patients in many countries worldwide. The prevalence seems to range from ~1:600 and ~1:700, respectively, in the Bukhara Jews and Uzbek Jews in Israel, to ~1:1000 in the French-Canadian population in Quebec, to ~1:100,000 in France (Blumen et al. 2009. PubMed ID: 19704078; Trollet et al. 2014. PubMed ID: 20301305). Nearly all cases of OPMD are caused by expansions within a GCG/GCA repeat that encodes a polyalanine tract immediately downstream of the translational start codon in the first exon of the PABPN1 gene (Brais et al. 1998. PubMed ID: 9462747; van der Sluijs et al. 2003. PubMed ID: 12673802; Trollet et al. 2014. PubMed ID: 20301305). The normal structure of the repeat is as follows, with both GCG and GCA encoding the amino acid alanine:
(GCG)6 (GCA)3 (GCG)
The total number of alanine residues encoded by the full repeat region is what defines an expanded repeat, and thus this region is typically referred to as (GCN)X/AlaX, where X is the total number of codons encoding alanine. The PABPN1 (GCN) repeat has been shown to be both meiotically and mitotically stable, and thus genetic anticipation has not been observed in this disorder (Robinson et al. 2005. PubMed ID: 15645184; Trollet et al. 2014. PubMed ID: 20301305).
A normal allele contains a total of 10 alanine codons, and is described as (GCN)10/Ala10. Fully penetrant expanded alleles have been reported to range between (GCN)12/Ala12 and (GCN)18/Ala18 (Trollet et al. 2014. PubMed ID: 20301305). The (GCN)11/Ala11 allele has been reported at a frequency of ~1-2% in North American, European and Japanese populations, and was initially considered to be a clinically benign polymorphism when in the heterozygous state with a normal (GCN)10/Ala10 allele (Trollet et al. 2014. PubMed ID: 20301305). However, based on recent studies, the (GCN)11/Ala11 allele is now considered to be a reduced penetrance dominant OPMD allele. Some patients heterozygous for the (GCN)11/Ala11 allele and a normal (GCN)10/Ala10 allele do not show clinical features, while others present with a later-onset, milder form of OPMD (Trollet et al. 2014. PubMed ID: 20301305; Richard et al. 2015. PubMed ID: 27858728; Richard et al. 2017. PubMed ID: 28011929).
Genotype-phenotype correlation between repeat size and clinical presentation has recently been described (Richard et al. 2017. PubMed ID: 28011929). Patients with a greater repeat size tended to have an earlier onset of clinical symptoms. In addition, in those with expansions greater than (GCN)14/Ala14, proximal weakness of the pelvic girdle was often observed at the time of diagnosis. Patients homozygous for a repeat of (GCN)12/Ala12 or greater tended to present earlier with more severe and/or additional clinical features, such as cognitive impairment, progressive gaze limitation, limb-girdle weakness leading to wheelchair use or bed confinement, and psychiatric features; they may also have a shortened lifespan (Blumen et al. 2009. PubMed ID: 19704078). Patients homozygous for the (GCN)11/Ala11 allele tend to present later with a mild clinical course (Trollet et al. 2014. PubMed ID: 20301305; Richard et al. 2017. PubMed ID: 28011929). Lastly, ~20% of patients compound heterozygous for a full penetrance allele and the (GCN)11/Ala11 reduced penetrance allele present earlier and with more severe features than those compound heterozygous for a full penetrance allele and a normal (GCN)10/Ala10 allele (Trollet et al. 2014. PubMed ID: 20301305; Richard et al. 2017. PubMed ID: 28011929). Therefore, the (GCN)11/Ala11 allele is considered to act as a modifier to increase the severity of disease in these compound heterozygous patients.
In addition to the known (GCN) repeat expansion, one nearby single nucleotide change has also been reported to be causative for OPMD. This change (c.35G>C) occurred within the codon immediately downstream of the last (GCG) codon of the PABPN1 exon 1 (GCN) repeat. The result of this nucleotide change was the substitution of an alanine for a glycine (p.Gly12Ala). Two additional alanine codons occur downstream of the glycine codon; thus, the c.35G>C sequence variant lead to the extension of the polyalanine tract, resulting in a total of 13 contiguous alanines (Robinson et al. 2006. PubMed ID: 16648376).
The PABPN1 gene (14q11.2) contains 7 coding exons and encodes the uqibuitously expressed poly-adenine-binding protein nuclear 1 protein. This protein plays a role in the regulation of poly(A) tail length, and may also assist in transferring poly(A) RNA out of the nucleus (Trollet et al. 2014. PubMed ID: 20301305; Abbassi-Daloii et al. 2017. PubMed ID: 28649424). It has also been shown to have several protein binding partners that play different roles in the cell, suggesting other less well-understood roles for the PABPN1 protein (Trollet et al. 2014. PubMed ID: 20301305).
Clinical Sensitivity - Expansion Assay
The sensitivity of this test is expected to be ~100% for individuals with confirmed oculopharyngeal muscular dystrophy OPMD, as the disorder is caused solely by expansion of the (GCN) repeat within exon 1 of the PABPN1 gene, or rarely by sequence variants near the (GCN) repeat region (Brais et al. 1998. PubMed ID: 9462747; Robinson et al. 2006. PubMed ID: 16648376; Richard et al. 2017. PubMed ID: 28011929).
Clinical sensitivity in patients with a clinical diagnosis of suspected oculopharyngeal muscular dystrophy (OPMD) of an unknown genetic etiology is expected to be lower. In two larger cohorts (242 and 900 patients, respectively) approximately 35-40% of patients were found to have an expansion of the (GCN) repeat in exon 1 of PABPN1 (Robinson et al. 2005. PubMed ID: 15645184; Richard et al. 2017. PubMed ID: 28011929).
Testing Strategy
A combination of amplicon-length analysis and bidirectional Sanger sequencing is used as a screening method for the presence or absence of a pathogenic GCN trinucleotide repeat expansion located in the first exon of PABPN1 (Brais et al. 1998. PubMed ID: 9462747; Trollet et al. 2014. PubMed ID: 20301305).
Amplicon-length analysis is also performed essentially as previously described (Brais et al. 1998. PubMed ID: 9462747; Richard et al. 2017. PubMed ID: 28011929). Test controls included DNA samples from individuals with a repeat expansion in PABPN1 in either the full penetrance or reduced penetrance size range, as well as healthy individuals.
This test is designed to detect pathogenic expansions within the (GCN) repeat in exon 1 of the PABPN1 gene, as well as sequence variants within this region.
Indications for Test
Testing should be considered for patients presenting with clinical features of oculopharyngeal muscular dystrophy (OPMD), including ptosis, dysphagia, and proximal limb muscle weakness. This test may also be considered for patients with a family history of OPMD.
Patients of Bukharan Jewish descent with suspected OPMD as well as additional visual features may consider sequencing the NRL gene in addition to performing molecular testing of PABPN1 (Newman et al. 2016. PubMed ID: 27732723; Braverman et al. 2017. PubMed ID: 28590779).
Testing should be considered for patients presenting with clinical features of oculopharyngeal muscular dystrophy (OPMD), including ptosis, dysphagia, and proximal limb muscle weakness. This test may also be considered for patients with a family history of OPMD.
Patients of Bukharan Jewish descent with suspected OPMD as well as additional visual features may consider sequencing the NRL gene in addition to performing molecular testing of PABPN1 (Newman et al. 2016. PubMed ID: 27732723; Braverman et al. 2017. PubMed ID: 28590779).
Gene
| Official Gene Symbol | OMIM ID |
|---|---|
| PABPN1 | 602279 |
| Inheritance | Abbreviation |
|---|---|
| Autosomal Dominant | AD |
| Autosomal Recessive | AR |
| X-Linked | XL |
| Mitochondrial | MT |
Disease
| Name | Inheritance | OMIM ID |
|---|---|---|
| Oculopharyngeal Muscular Dystrophy | AD | 164300 |
Citations 
- Abbassi-Daloii et al. 2017. PubMed ID: 28649424
- Blumen et al. 2009. PubMed ID: 19704078
- Brais et al. 1998. PubMed ID: 9462747
- Braverman et al. 2017. PubMed ID: 28590779
- Newman et al. 2016. PubMed ID: 27732723
- Richard et al. 2015. PubMed ID: 27858728
- Richard et al. 2017. PubMed ID: 28011929
- Robinson et al. 2005. PubMed ID: 15645184
- Robinson et al. 2006. PubMed ID: 16648376
- Trollet et al. 2014. PubMed ID: 20301305
- van der Sluijs et al. 2003. PubMed ID: 12673802
- van der Sluijs et al. 2016. PubMed ID: 27854203
Ordering/Specimens 
Ordering Options
We offer several options when ordering sequencing tests. For more information on these options, see our Ordering Instructions page. To view available options, click on the Order Options button within the test description.
myPrevent - Online Ordering
- The test can be added to your online orders in the Summary and Pricing section.
- Once the test has been added log in to myPrevent to fill out an online requisition form.
- PGnome sequencing panels can be ordered via the myPrevent portal only at this time.
Requisition Form
- A completed requisition form must accompany all specimens.
- Billing information along with specimen and shipping instructions are within the requisition form.
- All testing must be ordered by a qualified healthcare provider.
For Requisition Forms, visit our Forms page
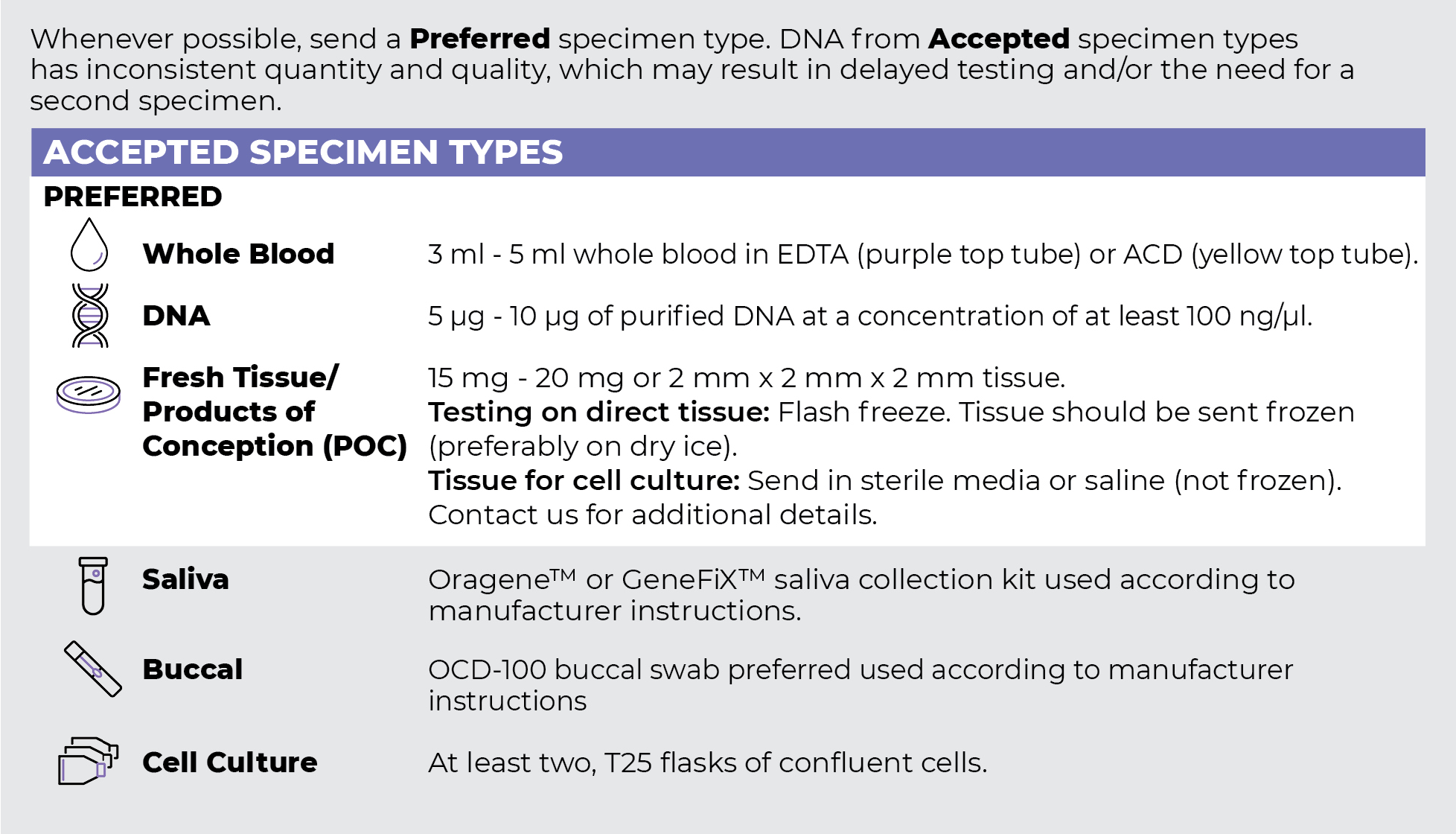
Specimen Types
Specimen Requirements and Shipping Details
ORDER OPTIONS
View Ordering Instructions1) Select Test Type
2) Select Additional Test Options
No Additional Test Options are available for this test.

